Limiting Reactant Organic Chemistry Lab . The concept of a limiting reactant in the preparation of brownies. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. define and determine theoretical yields, actual yields, and percent yields. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. Identify a limiting reagent from a set of reactants. For a chemist, the balanced. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
from studylib.net
The concept of a limiting reactant in the preparation of brownies. define and determine theoretical yields, actual yields, and percent yields. For a chemist, the balanced. Identify a limiting reagent from a set of reactants. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
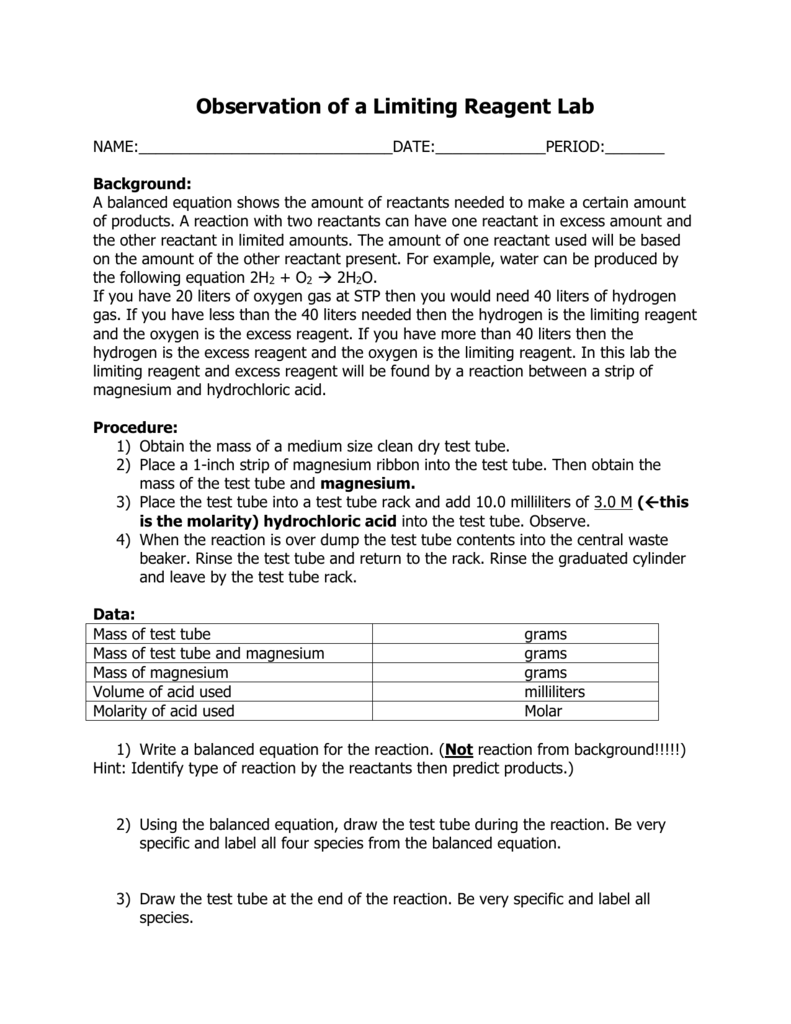
Observation of a Limiting Reagent Lab
Limiting Reactant Organic Chemistry Lab Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. define and determine theoretical yields, actual yields, and percent yields. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. For a chemist, the balanced. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. The concept of a limiting reactant in the preparation of brownies. Identify a limiting reagent from a set of reactants.
From studylib.net
Experiment 4 Limiting Reactant Limiting Reactant Organic Chemistry Lab define and determine theoretical yields, actual yields, and percent yields. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. The concept of a limiting. Limiting Reactant Organic Chemistry Lab.
From www.geeksforgeeks.org
Limiting Reagent Definition, Methods, Solved Examples, and FAQs Limiting Reactant Organic Chemistry Lab The concept of a limiting reactant in the preparation of brownies. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. For a chemist, the balanced. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Utilising stoichiometry coefficients and molar. Limiting Reactant Organic Chemistry Lab.
From www.thoughtco.com
Calculating Limiting Reactant of a Chemical Reaction Limiting Reactant Organic Chemistry Lab The concept of a limiting reactant in the preparation of brownies. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. define and determine theoretical yields, actual yields, and percent yields. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. in chemical reactions involving multiple reactants, the concept. Limiting Reactant Organic Chemistry Lab.
From studylib.net
Observation of a Limiting Reagent Lab Limiting Reactant Organic Chemistry Lab determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. Identify a limiting reagent from a set of reactants. Utilising stoichiometry coefficients and molar mass calculations, determining. Limiting Reactant Organic Chemistry Lab.
From alaniferscannon.blogspot.com
How to Determine Limiting Reactant Limiting Reactant Organic Chemistry Lab in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Identify a limiting reagent from a set of reactants. For a chemist, the balanced. Utilising stoichiometry. Limiting Reactant Organic Chemistry Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free download ID421375 Limiting Reactant Organic Chemistry Lab in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. The concept of a limiting reactant in the preparation of brownies. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. define and determine theoretical. Limiting Reactant Organic Chemistry Lab.
From www.studocu.com
Zn Cl2 Limiting Reactant Lab Limiting Reactant Lab When a reaction occurs, an equation for the Limiting Reactant Organic Chemistry Lab Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. The concept of a limiting reactant in the preparation of brownies. For a chemist, the balanced. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Identify a limiting reagent from a set of reactants. . Limiting Reactant Organic Chemistry Lab.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Limiting Reactant Organic Chemistry Lab Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. For a chemist, the balanced. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. The concept of a limiting reactant in the preparation of brownies. define and determine theoretical yields, actual yields, and percent yields. Use mole ratios. Limiting Reactant Organic Chemistry Lab.
From www.chegg.com
Solved Limiting Reactant Lab Chem. 101 Limiting Reactant Lab Limiting Reactant Organic Chemistry Lab determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Identify a limiting reagent from a set of reactants. Utilising stoichiometry coefficients and molar mass calculations,. Limiting Reactant Organic Chemistry Lab.
From www.transformationtutoring.com
How To Find Limiting Reactant, Theoretical Yield And Amount Of Excess Reagent Left (with examples) Limiting Reactant Organic Chemistry Lab The concept of a limiting reactant in the preparation of brownies. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance.. Limiting Reactant Organic Chemistry Lab.
From www.studocu.com
Copy of 3.8 Limiting Reactant Assignment Chemistry I 3 Limiting Reactants Assignment Limiting Reactant Organic Chemistry Lab in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. define and determine theoretical yields, actual yields, and percent yields. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient. Limiting Reactant Organic Chemistry Lab.
From www.studocu.com
Limiting Reactant Organic Chemistry Tutor based notes 12 atoms OF zinc , 8 molecules of HC Limiting Reactant Organic Chemistry Lab Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. The concept of a limiting reactant in the preparation of brownies. define and determine theoretical yields, actual yields, and percent yields. For a chemist, the balanced. determine which. Limiting Reactant Organic Chemistry Lab.
From www.studocu.com
Limiting reactant and reaction yield Organic Chemistry Lab II UB Studocu Limiting Reactant Organic Chemistry Lab define and determine theoretical yields, actual yields, and percent yields. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. For a chemist, the balanced. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. determine which reactant is. Limiting Reactant Organic Chemistry Lab.
From www.slideserve.com
PPT Laboratory 08 LIMITING REACTANT LAB PowerPoint Presentation, free download ID421375 Limiting Reactant Organic Chemistry Lab Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. define and determine theoretical yields, actual. Limiting Reactant Organic Chemistry Lab.
From www.wisegeek.com
In Chemistry, what is a Limiting Reactant? (with pictures) Limiting Reactant Organic Chemistry Lab Utilising stoichiometry coefficients and molar mass calculations, determining limiting reagents. Identify a limiting reagent from a set of reactants. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. define and determine theoretical yields, actual yields, and percent yields. Use mole ratios to calculate the number of moles of. Limiting Reactant Organic Chemistry Lab.
From ceewanzz.blob.core.windows.net
What Are Limiting Reactants at Jose Cruse blog Limiting Reactant Organic Chemistry Lab The concept of a limiting reactant in the preparation of brownies. determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. define and determine theoretical yields, actual yields, and percent yields. Use mole ratios to calculate the number of moles of product that can be. Limiting Reactant Organic Chemistry Lab.
From www.youtube.com
REACTANTS INVOLVING LIMITING REACTANT Chemistry Animation YouTube Limiting Reactant Organic Chemistry Lab The concept of a limiting reactant in the preparation of brownies. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant. For a chemist, the balanced. determine which reactant is. Limiting Reactant Organic Chemistry Lab.
From www.youtube.com
Limiting Reactant Practice Problems YouTube Limiting Reactant Organic Chemistry Lab determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. For a chemist, the balanced. define and determine theoretical yields, actual yields, and percent yields. in chemical reactions involving multiple reactants, the concept of the limiting reactant is crucial as it is the substance.. Limiting Reactant Organic Chemistry Lab.